Study data collection, automated.
Automatically generate eCRFs and source documents from your protocol, automate source data entry to enable truly remote monitoring, and ensure full 21 CFR Part 11 compliance.
trusted by
Everything you need to build, capture, and monitor your clinical data.
Legacy systems are just digital paper. Harbor automates the busywork, from the moment you draft the protocol to the final database lock, so you can focus on the science.
Demographics
Demographics
Protocol-to-EDC in Minutes
Stop waiting weeks for database builds. Harbor's Magic Build engine parses your protocol PDF and automatically generates CRFs and source documents instantly.
YSI 2300 STAT PLUS
In-Clinic Day
Blood Draw #1
Automated Data Capture
Sites upload source documents, and Harbor's AI extracts the data directly into the EDC—cutting transcription time by 90% and reducing errors.
Remote Monitoring
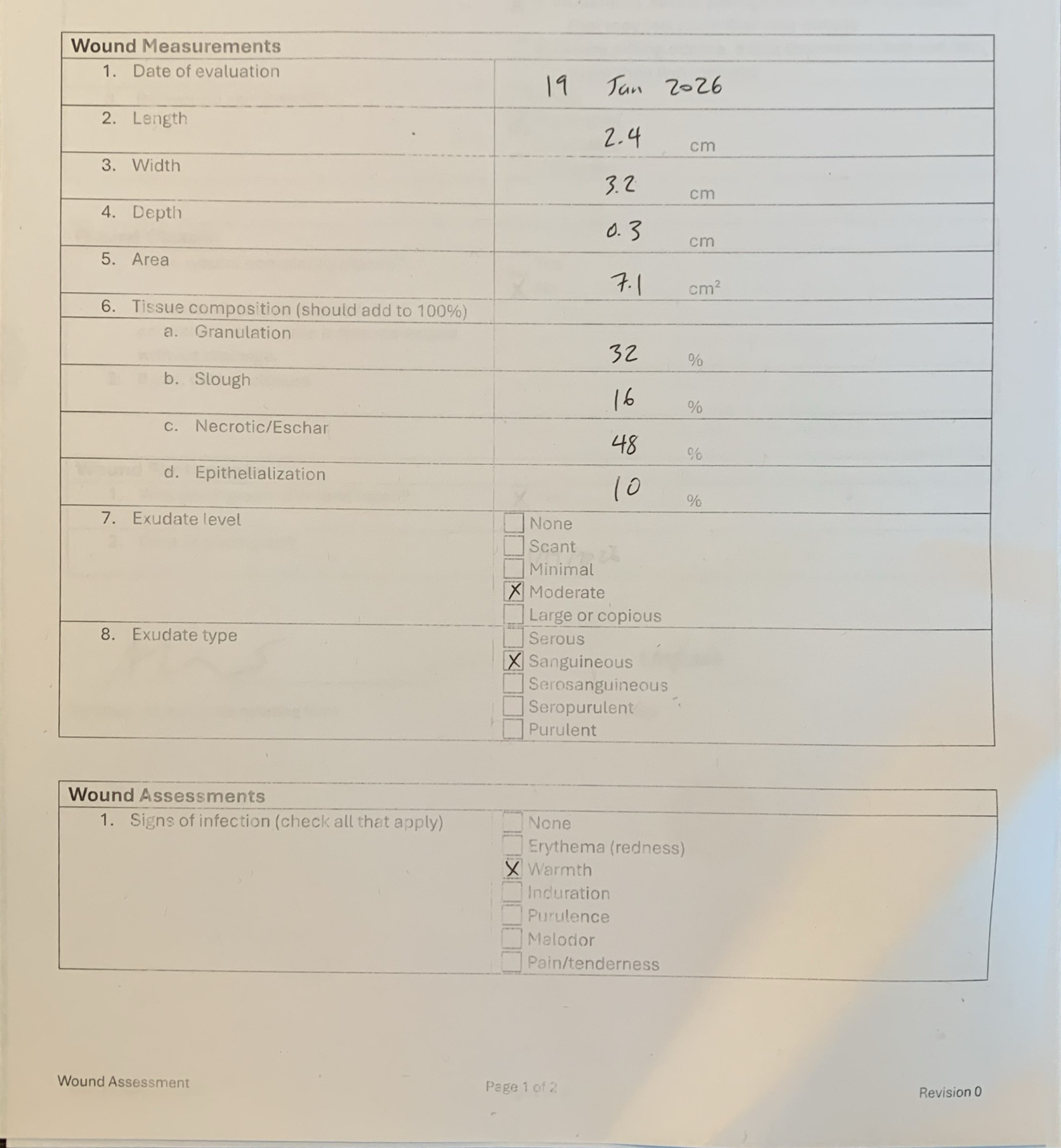
Risk-Based, Remote Monitoring
View source documents side-by-side with EDC data from anywhere in the world. Each data point is scored for extraction confidence and clinical relevance, enabling centralized and risk-based monitoring without forcing sites to change their workflows.
Built for efficiency
Unblock your trial at every stage.
Legacy EDCs create bottlenecks with slow builds, manual entry, and delayed data access. Harbor removes the friction, compressing months of busywork into minutes of automation.
To generate a full EDC build. Upload your protocol PDF and get a draft CRF and source document packet instantly. No more 8-week build delays.
Reduction in manual data entry. Sites upload source docs, and AI handles the transcription. Turn 2 hours of typing into 15 minutes of review.
Data visibility. Zero latency. See source data the moment it is uploaded, rather than waiting 7-10 days for site entry.
Creating source documents and CRFs used to be a standalone, two-month project for us. With Harbor, the system generated them automatically as part of the EDC build, cutting that entire process down to just two days. It streamlined our study startup phase significantly.

Johnny Chen
Founder & CEO, BioDynamik
Security
Security is our baseline, not a feature.
Harbor is architected from the ground up to meet the most stringent requirements of the FDA, HIPAA, and sponsor IT teams.
Compliance Stack
We do not just support compliance; we enforce it. Harbor creates a validated environment with immutable audit trails, eSignatures, and role-based access controls by default.
Validation Package Included
Skip the 6-month validation headache. We provide a comprehensive Verification and Validation package with every license, including risk and traceability matrices.
Military-Grade Encryption
Your data is encrypted at rest using AES-256 and in transit via TLS 1.2+. Each study is hosted in logically isolated databases to prevent cross-contamination.
Business Continuity
Our infrastructure runs on multi-zone redundancy with automated daily backups. In the event of a catastrophic regional failure, your trial data is safe and recoverable within minutes.
Zero Data Retention
Your organization’s data is only used for the clinical trials you run. We maintain zero-data retention and enforce business associate agreements (BAA) with all partners to ensure privacy, security, and compliance.
Questions & Answers
One platform. All the features.
Academic & Non-Profit
Free
University research and investigator-initiated trials.
Full AI suite (Build, Capture, Monitor)
Unlimited users and patients
Standard support (email)
Standard export
Commercial Study
Starting at $2,000/mo
Growing teams needing collaboration and insights
Everything in Academic
21 CFR Part 11 validation package
Priority support (Slack channel)
Training for sites and CRAs
Guaranteed uptime SLA
Enterprise / CRO
Volume pricing
CROs and sponsors with 3+ active trials.
Everything in Commercial
Master service agreement (MSA)
SSO / SAML
CTMS / eTMF integrations
Dedicated customer success manager
Everything Included
Magic Build: Protocol-to-CRF automation.
Magic Capture: AI source data entry.
Magic Monitor: Confidence scores to enable risk-based and remote monitoring.
ePROs: Patient data entry.
Storage: Unlimited source document uploads.
Ready to modernize your clinical data collection?
Stop settling for slow builds and manual entry. Schedule a personalized demo to discuss your specific protocol requirements and see how Harbor can reduce your trial timelines.